A Better Approach for Treating DED
Deliver the Long-lasting Relief You and Your Patients Need
Treating MGD-related DED Continues to Pose Challenges
Removing obstruction is critical
Most eye care professionals (ECPs) agree that obstruction removal should be the first-line treatment for meibomian gland dysfunction (MGD).1
Patients don’t recognize that MGD is a chronic disease
Patients commonly stop self-administered treatments like warm compresses and drops when symptoms improve, allowing them to worsen once again.2
Learn more about the TearCare® System.
TearCare® is currently available in the U.S. only. For future information about international capabilities, click here.
TearCare®, a Solution Designed for MGD Relief
TearCare® is a heat and expression device that takes patient noncompliance out of the equation.
MGD Treatment With the Power to Last
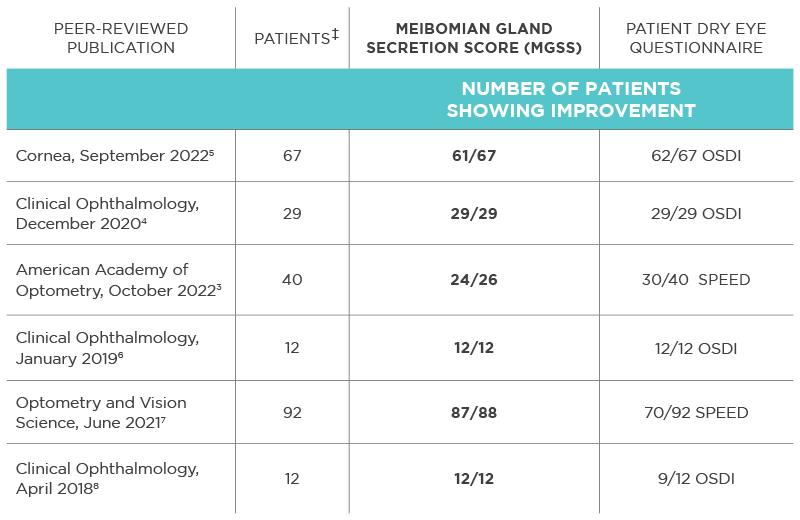
Significant improvements*
Improvement in
1 week4
Durable through
12 months3
Advancing the Future of MGD Treatment
Smart. Convenient. Long-lasting. The unique features of the TearCare® System allow ECPs and their patients to experience an MGD treatment like no other.
SmartLids™
- Single-use with a universal fit
- Consistent therapeutic heat (41° C to 45° C) for up to 15 minutes
- Open-eye design for natural blinking during treatment

SmartHub™
- Sensor-driven therapeutic consistency
- Precisely manages therapeutic heat level
- Rechargeable and highly portable

Clearance Assistant™
- Single-use, stainless steel forceps
- Controlled expression of obstructed meibum
- Provides visual confirmation of expression
Support for You and Your Patients Every Step of the Way
Patient education resources
A library of TearCare® product and patient photos, patient testimonial videos, and educational videos for your own marketing purposes.
Peer-to-peer collaboration
- TearCare® Education and Mentorship (TEaM): One-on-one support programs and live events with experts to receive guidance while using TearCare®
- DocMatter: An online platform to ask questions and learn helpful information on MGD and TearCare® from fellow eyecare providers
Ask your TearCare® sales representative about all the resources we have to offer.
Patient Satisfaction Is Our Priority
8 out of 10 patients say TearCare® is comfortable and would likely recommend it to family and friends.†

We care enough about the success of your patients’ treatment to put a guarantee behind the TearCare® System, a TearCare® Promise.
This program ensures trust and peace of mind for you and your patients.
References:
* In retrospective 1-month and 12-month analyses of the durability of response in the suppression of dry eye signs and symptoms after a single TearCare® treatment. Effectiveness was assessed in the 1-month study as mean change from baseline in tear break-up time (TBUT), Ocular Surface Disease Index (OSDI), total Meibomian Gland Secretion Score (MGSS), and corneal/conjunctival staining. Symptom severity was measured by Standard Patient Evaluation of Eye Dryness questionnaire (SPEED) score, and sign improvement was measured by mean meibomian gland expression (MGE) scores in the 12-month analysis. Findings were found to be clinically and statistically significant.
† Response results from opt-in patient rebate surveys administered by TearCare® from January 2021 through November 2021, Data on file. Top 2 box responses (very/somewhat comfortable/most definitely/probably).
‡ The 2018 and 2019 studies by Badawi show results from the same patient group.
- Data on file. Sight Sciences.
- Hunter J, Chatila K. Dry Eye Device Market Understanding And Motivators. Presentation of Findings; 2022.
- Chester T, Ferguson T, Chester E. TearCare® for the Treatment of Meibomian Gland Dysfunction: A Single Center Trial Measuring Durability of Treatment. American Academy of Optometry. 2022.
- Karpecki P, Wirta D, Osmanovic S, et al. A prospective, post-market, multicenter trial (CHEETAH) suggested TearCare® System as a safe and effective blink assisted eyelid device for the treatment of dry eye disease. Clin Ophthalmol. 2020;14:4551-4559. doi:10.2147/OPTH.S285953
- Gupta PK, Holland EJ, Hovanesian J, et al. TearCare for the treatment of meibomian gland dysfunction in adult patients with dry eye disease: a masked randomized controlled trial. Cornea. 2022;41(4):417-426. doi:10.1097/ICO.0000000000002837
- Badawi D. TearCare® system extension study: evaluation of the safety, effectiveness, and durability through 12 months of a second TearCare® treatment on subjects with dry eye disease. Clin Ophthalmol. 2019;13:189-198. doi:10.2147/OPTH.S191588
- Chester T. A single-center retrospective trial of a blink-assisted eyelid device in treating the signs and symptoms of dry eye. Optom Vis Sci. 2021;98(6):605-612. doi:10.1097/OPX.0000000000001711
- Badawi D. A novel system, TearCare®, for the treatment of the signs and symptoms of dry eye disease. Clin Ophthalmol. 2018;12:683-694. doi:10.2147/OPTH.S160403
Indications for Use: The TearCare® System is intended for the application of localized heat therapy in adult patients with evaporative dry eye disease due to meibomian gland dysfunction (MGD), when used in conjunction with manual expression of the meibomian glands.
The TearCare® System may not be right for everyone. Please see Instructions for Use or visit TearCare.com for Contraindications, Warnings, Precautions and Adverse Events.
Indications for Use: The TearCare® Clearance Assistant is indicated to express meibum from meibomian glands.

4040 Campbell Ave, Suite 100
Menlo Park CA 94025
© 2023 Sight Sciences, Inc. 7/23 TC-1697-US.v3
TearCare®, SmartLids™, SmartHub™, Clearance Assistant™, Sight Sciences, and the Sight Sciences logo are trademarks or registered trademarks of Sight Sciences.